The bustling cityscape, a symphony of honking cars and chattering crowds, always seemed to pulsate with a relentless energy. Yet, amidst the chaos, there was a hidden order, a delicate balance of forces that kept everything moving in a predictable way. And as I stood watching the city unfold, I realized that even the most complex systems, from bustling metropolitan centers to the tiny reactions within our cells, operate on a principle of equilibrium. This is the same principle that governs the flow of energy in chemical reactions, a principle we call “free energy.”

Image: yoshikafurukawa.blogspot.com
In the world of chemistry, free energy is a fundamental concept that helps us understand and predict how reactions will unfold. It’s the energy available to do work and a key driver for spontaneous processes. It not only tells us how a reaction is likely to proceed but also reveals the conditions where a reaction will favor the formation of products over reactants. In this blog post, we’ll delve into the fascinating world of work equilibrium and free energy with a focus on using POGIL (Process Oriented Guided Inquiry Learning) as a tool to enhance understanding.
Unraveling Work Equilibrium and Free Energy
Before we embark on our POGIL journey, it’s crucial to set a solid foundation of understanding about work equilibrium and free energy. These interconnected concepts underpin the very essence of how chemical reactions occur and how energy flows within systems.
Work equilibrium, simply put, describes a state where the rate of a forward reaction equals the rate of its reverse reaction. When a system reaches work equilibrium, there’s no net change in the concentrations of reactants and products. This doesn’t imply that the reaction has stopped; instead, it signifies an equal pace of forward and reverse reactions. This equilibrium state is often dynamically maintained, with molecules constantly transitioning between reactant and product forms.
Free energy, on the other hand, represents the energy available in a system to do useful work. It’s the energy that can drive a reaction to spontaneously proceed. The change in free energy (ΔG) during a reaction tells us whether a process is favorable or not. A negative ΔG indicates that the reaction will spontaneously proceed, releasing energy to the surroundings – creating a thermodynamically favorable reaction. A positive ΔG indicates that the reaction requires an input of energy to occur. The two concepts, work equilibrium and free energy, are intimately connected. Work equilibrium is the state reached when a reaction’s free energy change becomes zero.
Understanding Work Equilibrium and Free Energy Through POGIL Activities
POGIL (Process Oriented Guided Inquiry Learning) is a powerful pedagogical approach that actively engages students in the learning process. POGIL activities are designed to encourage students to think critically, analyze data, and draw their own conclusions. This hands-on, collaborative approach transforms learning from passive absorption of information to active exploration and discovery.
In the context of work equilibrium and free energy, POGIL can be a particularly effective tool for fostering deeper understanding. Let’s explore how POGIL activities can be integrated to help students grasp these abstract concepts:
- Introduction to Free Energy and Enthalpy: POGIL activities can start with basic definitions and examples. Students can be presented with data on different chemical reactions and asked to calculate the change in Gibbs free energy (ΔG) based on the change in enthalpy (ΔH) and entropy (ΔS). This helps them understand the relationship between various thermodynamic parameters and the spontaneity of a reaction.
- Work Equilibrium and the Equilibrium Constant: POGIL can help students explore the connection between free energy and the equilibrium constant (K). Activities could involve analyzing experimental data to determine the value of K for various reactions. By plotting the relationship between ΔG and K, students can visualize the impact of free energy on the position of equilibrium.
- Le Chatelier’s Principle and Equilibrium Shifts: POGIL activities can help students understand how changes in conditions, such as temperature or pressure, can shift the equilibrium position of a reaction. Students could be asked to predict the direction of equilibrium shift based on various changes in conditions and then design experiments to verify their predictions.
- Free Energy and the Coupling of Reactions: The POGIL approach allows students to delve into how reactions can be coupled to drive a non-spontaneous process. Students could analyze data on coupled reactions and calculate the overall free energy change. This helps them understand how reactions with positive ΔG can be driven by reactions with negative ΔG.
- Real-World Applications of Work Equilibrium and Free Energy: POGIL can connect theoretical concepts to real-world applications. For instance, students could explore the role of free energy in biological systems, such as the energy transfer in cellular respiration or the equilibrium of protein folding. They can even be presented with scenarios involving industrial processes like the production of ammonia or the synthesis of certain pharmaceuticals, where understanding work equilibrium and free energy is crucial.
Tips and Expert Advice: Mastering Work Equilibrium and Free Energy
Navigating the world of work equilibrium and free energy can feel overwhelming at first. But with the right approach, you can master these concepts and gain a deeper understanding of chemical reactions. Here are some tips and expert advice to guide your learning journey:
Embrace Visual Aids: Work equilibrium and free energy concepts can be abstract. Use visual aids like graphs, diagrams, and models to help you visualize these concepts. The visual representation can make it easier to grasp the relationship between various factors.
Practice, Practice, Practice: Like any skill, understanding work equilibrium and free energy requires practice. Work through numerous examples and problems from your textbook or online resources. This repeated exposure will help you solidify your understanding and build confidence in applying the concepts.
Don’t Hesitate to Ask for Help: Don’t be afraid to seek clarification from your teachers or peers. Chemistry can be challenging, but discussing concepts with others can help you clarify any confusions and gain new perspectives.
Connect with Real-World Applications: Understanding the relevance of these concepts in real-world scenarios will motivate your learning. Explore how work equilibrium and free energy impact various fields, from medicine to environmental science, to enhance your appreciation of the subject.
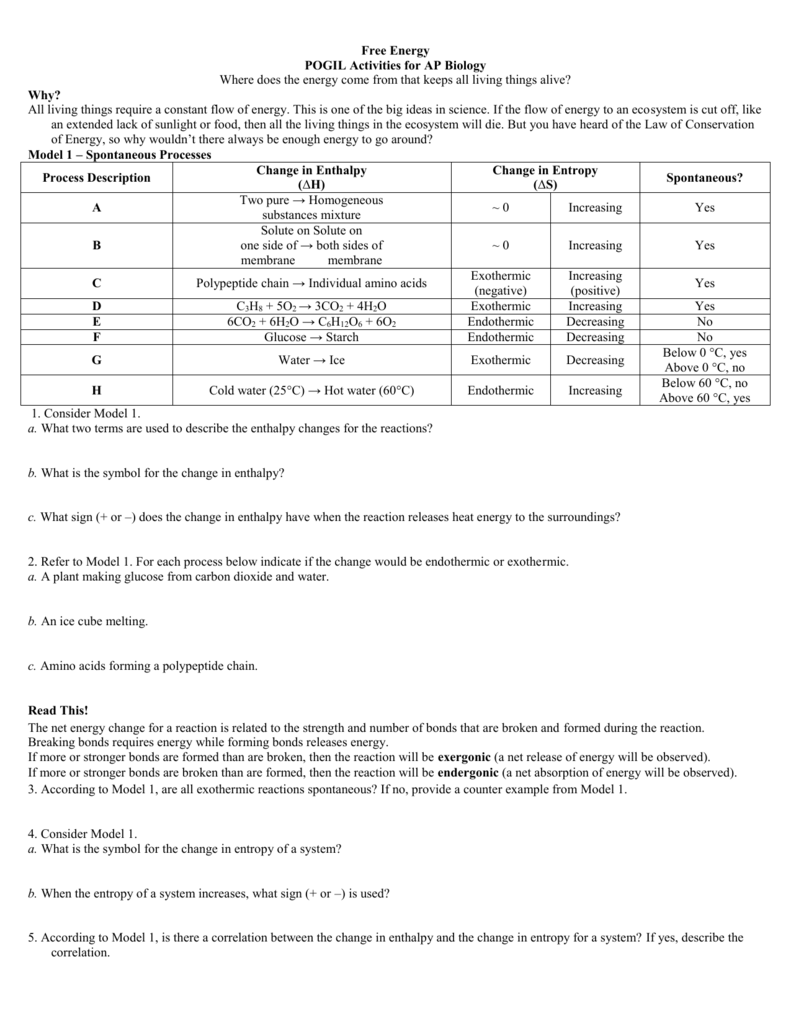
Image: studylib.net
Frequently Asked Questions
Q1: What is the significance of the Gibbs Free Energy change (ΔG) in chemical reactions?
A: The Gibbs Free Energy change (ΔG) dictates the spontaneity of a chemical reaction. A negative ΔG indicates a spontaneous reaction, while a positive ΔG signifies a non-spontaneous reaction requiring an energy input.
Q2: How can I determine the equilibrium constant (K) for a reaction using ΔG?
A: The relationship between ΔG and K is defined by the equation ΔG = -RTlnK, where R is the gas constant and T is the temperature in Kelvin. By knowing the ΔG, you can calculate the equilibrium constant.
Q3: What is the significance of Le Chatelier’s Principle in understanding work equilibrium?
A: Le Chatelier’s Principle states that a system at equilibrium will shift to relieve stress. In terms of work equilibrium, changing conditions (temperature, pressure, or concentration) will cause the system to shift to re-establish equilibrium.
Q4: How are coupled reactions important in biological systems?
A: Coupled reactions involve linking a favorable reaction with a non-favorable reaction. In biological systems, this allows energy released from favorable reactions to drive non-favorable reactions, enabling essential processes like ATP synthesis in cellular respiration.
Work Equilibrium And Free Energy Pogil Answer Key
Conclusion
Work equilibrium and free energy are truly foundational concepts in chemistry. Understanding them allows us to not only predict the direction of reactions but also explore their impact on various fields, from biological processes to industrial applications. POGIL is a powerful tool that can help you delve deeper into these concepts and understand the driving forces of chemical change. We encourage you to explore POGIL activities and embrace the concepts of work equilibrium and free energy. Explore, learn, and discover!
Are you fascinated by the world of chemistry and the role of work equilibrium and free energy in driving chemical reactions? If so, let’s continue this conversation to delve deeper into applications and explore new insights together.






